|
|||||||


The core of FyMed's strengths lie in its discovery and research where we are translating advanced science into medicines with profound clinical impact.
We have a scientific team of highly experienced PhDs and MDs with long-standing record of excellence and diverse expertise in basic science, translational, and clinical research. Our R&D team is constantly working on cutting-edge technologies and late-breaking science that lays the foundation for the development of innovative therapies at FyMed. FyMed now supports clients with clinical trial management and research services to accelerate drug development. |
Timeline of R&D Developments
 2011: Launched CRO services for clinical trial support
2011: Launched CRO services for clinical trial supportFyMed introduced CRO services to enhance R&D capabilities through clinical trial management.  2012: Scientists at FyMed complete devpt of FY10 technology
2012: Scientists at FyMed complete devpt of FY10 technologyResults reveal unique NSAID target profiles.  2012: FyMed identifies new anti-inflammatory formulation
2012: FyMed identifies new anti-inflammatory formulationStudies show huge potential for new NSAID strategies.  2013: Comparative studies on FyMed NSAID candidates FY101C & FY103B
2013: Comparative studies on FyMed NSAID candidates FY101C & FY103BResults confirm profound clinical efficacy.  2013: Examination of FyMed's technologies in precision medicine
2013: Examination of FyMed's technologies in precision medicineMethods peer reviewed as revolutionary.  2014: Lead FyMed NSAID candidate FY103B
2014: Lead FyMed NSAID candidate FY103BStudies demonstrate superiority over celecoxib and naproxen.  2020: Supported a Phase II trial for a biotech partner
2020: Supported a Phase II trial for a biotech partnerFyMed collaborated with a biotech client to advance discovery research through clinical trials. |
Stages of R&D Development at FyMed
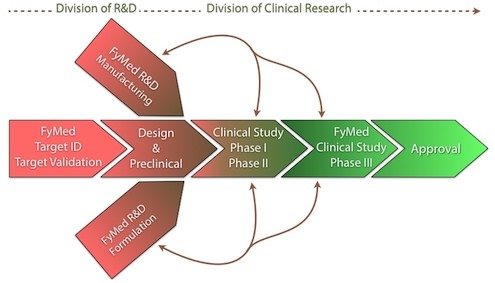 While fully independent, FyMed's R&D Division is very integrated into its Clinical Research Division, which supports clinical trial design and execution, translating molecular insights into clinical outcomes. Our technologies enable the identification of unique profiles for a particular disease and reveal the biological pathways it affects. This provides FyMed the additional capability to subclassify patients with different conditions and test these potential new drugs in patients who suffer from specific disease subtypes.
While fully independent, FyMed's R&D Division is very integrated into its Clinical Research Division, which supports clinical trial design and execution, translating molecular insights into clinical outcomes. Our technologies enable the identification of unique profiles for a particular disease and reveal the biological pathways it affects. This provides FyMed the additional capability to subclassify patients with different conditions and test these potential new drugs in patients who suffer from specific disease subtypes.This is an approach that bridges therapeutics and regulatory science, an area of increasing interest and high-priority to the medical community and the FDA. Contact us for clinical research partnerships.
Focus Areas
|
FyMed, Inc.
Leading Innovations in Precision Medicine ™
Pharmaceuticals:
103 Carnegie Center Dr, #300,
Princeton,
NJ
08540
Corporate:
3422 Old Capitol Rd, #1610,
Wilmington,
DE
19808
USA Tel:
+1 302-722-5010 |
Fax: +1 302-722-5011
UK Tel:
+44 1844 220 090 |
ask@fymedinc.com
Copyright © FyMed, Inc. |
All Rights Reserved





